Number Of Electrons In Iron
Atomic Number of Iron
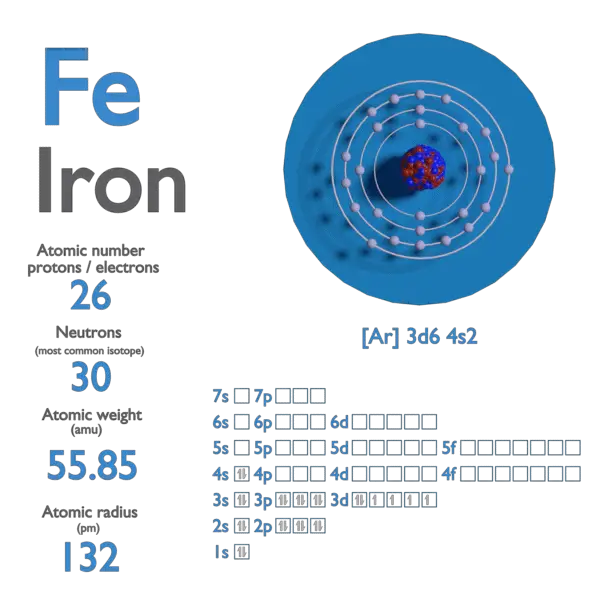
Fe is a chemic element with atomic number 26 which means in that location are 26 protons and 26 electrons in the atomic structure. The chemical symbol for Iron is Fe.
Since the number of electrons is responsible for the chemical behavior of atoms, theatomic number identifies the various chemical elements.
How does the atomic number determine the chemical beliefs of atoms?
Atomic Mass of Fe
Atomic mass of Iron is 55.845 u.
Note that each element may incorporate more isotopes. Therefore this resulting diminutive mass is calculated from naturally-occurring isotopes and their abundance.
The unit of mensurate for mass is theatomic mass unit (amu). Ane atomic mass unit is equal to 1.66 10 10-24 grams. One unified atomic mass unit isapproximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.
For12C, the diminutive mass is exactly 12u, since the atomic mass unit is defined from it. For other isotopes, the isotopic mass unremarkably differs and is normally within 0.1 u of the mass number. For example, 63Cu (29 protons and 34 neutrons) has a mass number of 63, and an isotopic mass in itsnuclear ground country is 62.91367 u.
In that location are two reasons for the departure betwixt mass number and isotopic mass, known as the mass defect:
- Theneutron is slightly heavier than theproton. This increases the mass of nuclei with more neutrons than protons relative to the diminutive mass unit calibration based on12C with equal numbers of protons and neutrons.
- The nuclear bounden energy varies betwixt nuclei. A nucleus with greater bounden energy has lower full free energy, and therefore alower mass according to Einstein's mass-free energy equivalence relationE =mc ii. For 63Cu, the diminutive mass is less than 63, so this must be the dominant factor.
The atomic mass number determines specially the atomic mass of atoms. The mass number is different for each dissimilar isotope of a chemic element.
How does the atomic mass decide the density of materials?
Density of Fe
Density of Iron is 7.874g/cmthree .

Typical densities of various substances at atmospheric pressure.
Density is defined as themass per unit book. It is anintensive belongings, which is mathematically defined as mass divided by book:
ρ = thousand/V
In other words, the density (ρ) of a substance is the total mass (1000) of that substance divided by the total volume (V) occupied by that substance. The standard SI unit is kilograms per cubic meter (kg/miii ). The Standard English unit ispounds mass per cubic pes (lbm/ftthree ).
See also: What is Density
Run across likewise: Densest Materials of the Earth

Iron – Backdrop Summary
| Element | Iron |
|---|---|
| Diminutive Number | 26 |
| Symbol | Fe |
| Element Category | Transition metal |
| Phase at STP | Solid |
| Atomic Mass [amu] | 55.845 |
| Density at STP [thousand/cm3] | seven.874 |
| Electron Configuration | [Ar] 3d6 4s2 |
| Possible Oxidation States | +ii,3 |
| Electron Analogousness [kJ/mol] | 15.seven |
| Electronegativity [Pauling scale] | ane.83 |
| 1st Ionization Energy [eV] | seven.9024 |
| Year of Discovery | unknown |
| Discoverer | unknown |
| Thermal properties | |
| Melting Indicate [Celsius scale] | 1538 |
| Boiling Point [Celsius scale] | 2861 |
| Thermal Electrical conductivity [Westward/g K] | 80.2 |
| Specific Heat [J/g K] | 0.44 |
| Heat of Fusion [kJ/mol] | xiii.8 |
| Heat of Vaporization [kJ/mol] | 349.6 |
Iron in Periodic Table
| Hydrogen i H | Helium 2 He | ||||||||||||||||||
| Lithium 3 Li | Beryllium four Exist | Boron 5 B | Carbon 6 C | Nitrogen seven N | Oxygen 8 O | Fluorine nine F | Neon 10 Ne | ||||||||||||
| And thendium 11 Na | Magnesium 12 Mg | Aluminium xiii Al | Silicon 14 Si | Phosphorus 15 P | Sulfur 16 S | Chlorine 17 Cl | Argon 18 Ar | ||||||||||||
| Potassium 19 K | Calcium 20 Ca | Scandium 21 Sc | Titanium 22 Ti | Vanadium 23 V | Chromium 24 Cr | Manganese 25 Mn | Iron 26 Fe | Cobalt 27 Co | Nickel 28 Ni | Copper 29 Cu | Zinc 30 Zn | Gallium 31 Ga | Germanium 32 Ge | Arsenic 33 As | Selenium 34 Se | Bromine 35 Br | Krypton 36 Kr | ||
| Rubidium 37 Rb | Strontium 38 Sr | Yttrium 39 Y | Zirconium 40 Zr | Niobium 41 Nb | Molybdenum 42 Mo | Technetium 43 Tc | Ruthenium 44 Ru | Rhodium 45 Rh | Palladium 46 Pd | Silver 47 Ag | Cadmium 48 Cd | Indium 49 In | Can 50 Sn | Antimony 51 Sb | Tellurium 52 Te | Iodine 53 I | Xenon 54 Xe | ||
| Caesium 55 Cs | Barium 56 Ba | Lanthanum 57 La | | Hafnium 72 Hf | Tantalum 73 Ta | Tungsten 74 W | Rhenium 75 Re | Osmium 76 Os | Iridium 77 Ir | Platinum 78 Pt | Aureate 79 Au | Mercury eighty Hg | Thallium 81 Tl | Pb 82 Pb | Bismuth 83 Bi | Polonium 84 Po | Astatine 85 At | Radon 86 Rn | |
| Francium 87 Fr | Radium 88 Ra | Actinium 89 Ac | | Rutherfordium 104 Rf | Dubnium 105 Db | Seaborgium 106 Sg | Bohrium 107 Bh | Hassium 108 Hs | Meitnerium 109 Mt | Darmstadtium 110 Ds | Roentgenium 111 Rg | Copernicium 112 Cn | Nipponium 113 Nh | Flerovium 114 Fl | Moscovium 115 Mc | Livermorium 116 Lv | Tennessine 117 Ts | Oganesson 118 Og | |
| | Cerium 58 Ce | Praseodymium 59 Pr | Neodymium 60 Nd | Promethium 61 Pm | Samarium 62 Sm | Europium 63 Eu | Gadolinium 64 Gd | Terbium 65 Tb | Dysprosium 66 Dy | Holmium 67 Ho | Erbium 68 Er | Thulium 69 Tm | Ytterbium 70 Yb | Lutetium 71 Lu | |||||
| | Thorium 90 Th | Protactinium 91 Pa | Uranium 92 U | Neptunium 93 Np | Plutonium 94 Pu | Americium 95 Am | Curium 96 Cm | Berkelium 97 Bk | Californium 98 Cf | Einsteinium 99 Es | Fermium 100 Fm | Mendelevium 101 Md | Nobelium 102 No | Lawrencium 103 Lr | |||||
–
–
–
Number Of Electrons In Iron,
Source: https://www.nuclear-power.com/Iron-atomic-number-mass-density/
Posted by: farmerlextre.blogspot.com


0 Response to "Number Of Electrons In Iron"
Post a Comment